Interpreting microstructures of stainless steel
Ferritic stainless steels do not respond to hardening. Their properties, however, can be influenced by cold working. They are magnetic at room temperature. The microstructure in the annealed condition consists of ferrite grains in which fine carbides are embedded. Ferritic steels used for machining contain a large amount of manganese sulfides to facilitate free cutting.
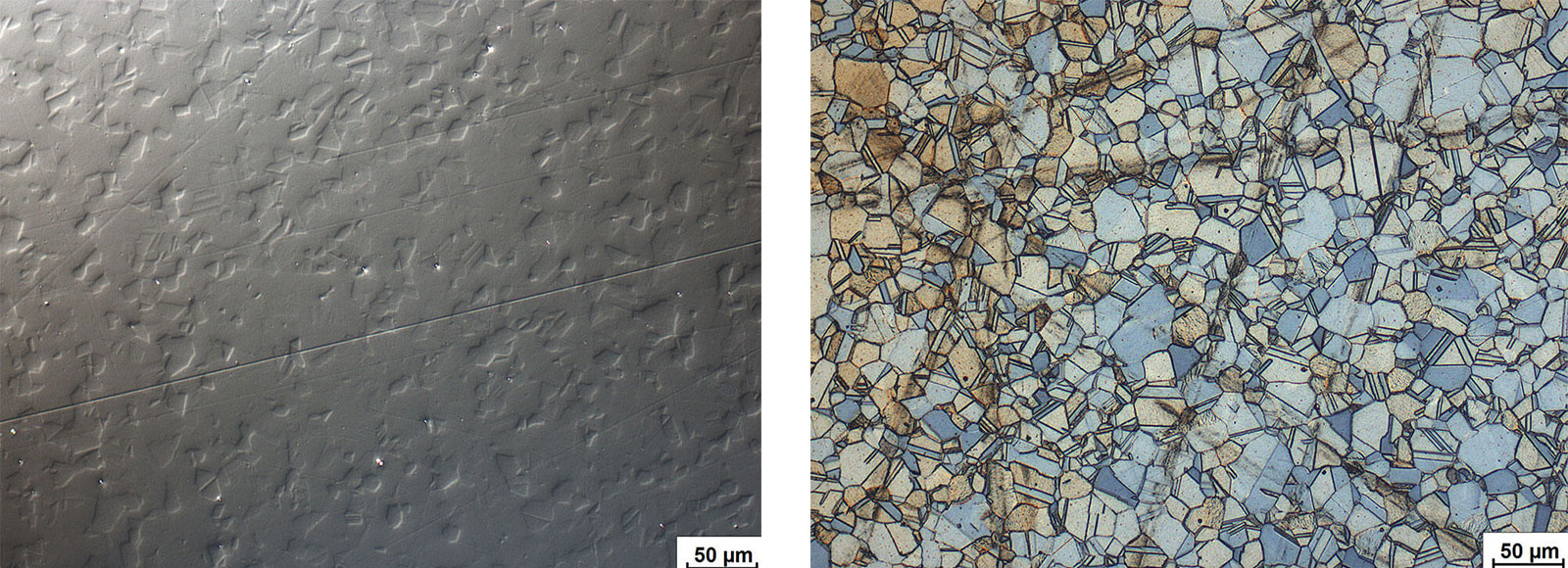
Martensitic stainless steels respond to heat treatment. Martensite is formed through rapid cooling. Properties can then be optimized by subsequent tempering treatment. The alloys are magnetic. Depending on the thermal treatment, the microstructure can range from a pure martensitic structure to fine-tempered martensite. Different alloys and various dimensions of semi-finished products require complex heat treatment temperatures and times.
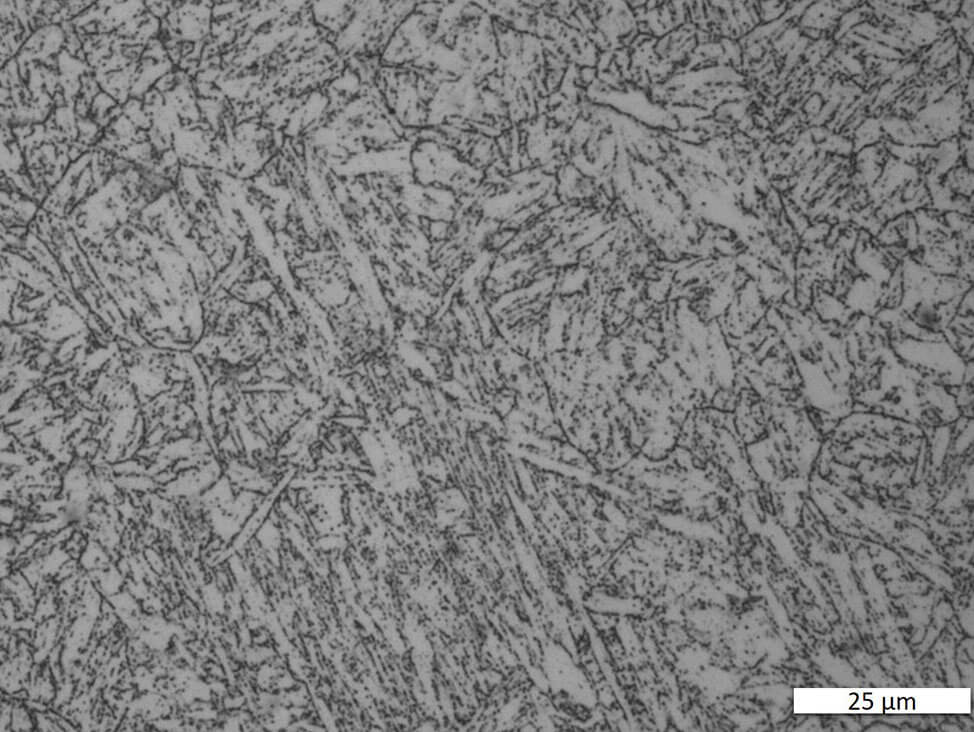 Fig. 6: Martensitic chrome steel, electrolytically polished and etched with A2. Bright field.
Fig. 6: Martensitic chrome steel, electrolytically polished and etched with A2. Bright field.
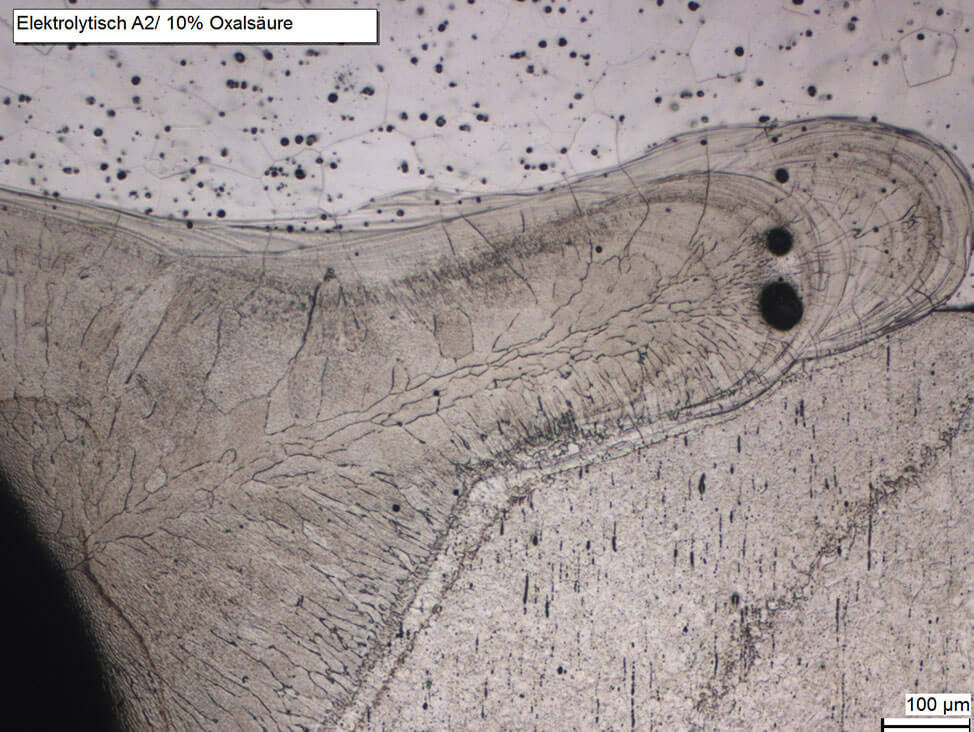
In some corrosion resistant steel welds, a certain amount of delta ferrite is needed to improve hot-crack resistance. However, delta ferrite is usually an unwanted phase, because the long annealing times of steel with a high chromium content can change the delta ferrite into the hard and brittle iron-chromium intermetallic sigma phase. Heating up to 1,050 °C and subsequent quenching removes the sigma phase and with it the embrittlement.
Austenitic stainless steels do not respond to thermal treatment. Instead, rapid cooling results in the production of their softest condition. In this state, they are non-magnetic and their properties are influenced by cold working. The microstructure of these steels consists of austenite grains, which may exhibit twinning.
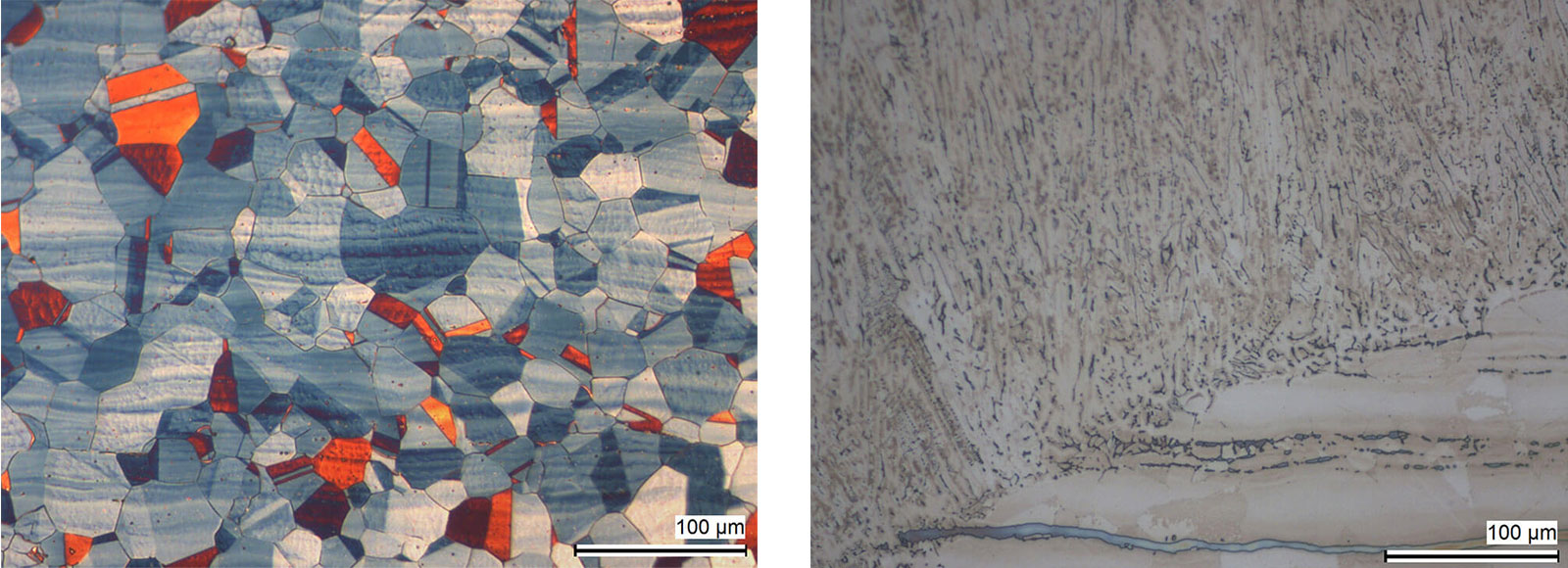 Fig. 7: Austenitic steel with twins and segeragtions. Colour etched with Lichtenegger and Bloech. DIC.
Fig. 8: Deltaferrite in an austenitic steel weld (small dark strings) and larger deltaferrite lines in weld part (blue-grey); electrolytically etched with 40 % aqueous sodium hydroxide solution. Bright field
Fig. 7: Austenitic steel with twins and segeragtions. Colour etched with Lichtenegger and Bloech. DIC.
Fig. 8: Deltaferrite in an austenitic steel weld (small dark strings) and larger deltaferrite lines in weld part (blue-grey); electrolytically etched with 40 % aqueous sodium hydroxide solution. Bright field
Exposing these steels to elevated temperatures in the region of 600-700 °C can result in the formation of complex carbides within the austenite grains. This leads to an impoverishment of chromium in the austenite solid solution, which increases the susceptibility to intergranular corrosion or oxidation.
 Fig. 9: Austenitc steel tube with twins and deformation from cold working; etched with 10 % oxalic acid, DIC
Fig. 9: Austenitc steel tube with twins and deformation from cold working; etched with 10 % oxalic acid, DIC
By reducing the carbon to below 0.015 % and adding small amounts of titanium, niobium or tantalum, the risk of intergranular corrosion is reduced, as these elements form carbides in preference to the chrome.
Delta ferrite can appear due to critical heat treatment conditions in martensitic steels or cold working of austenitic steels.
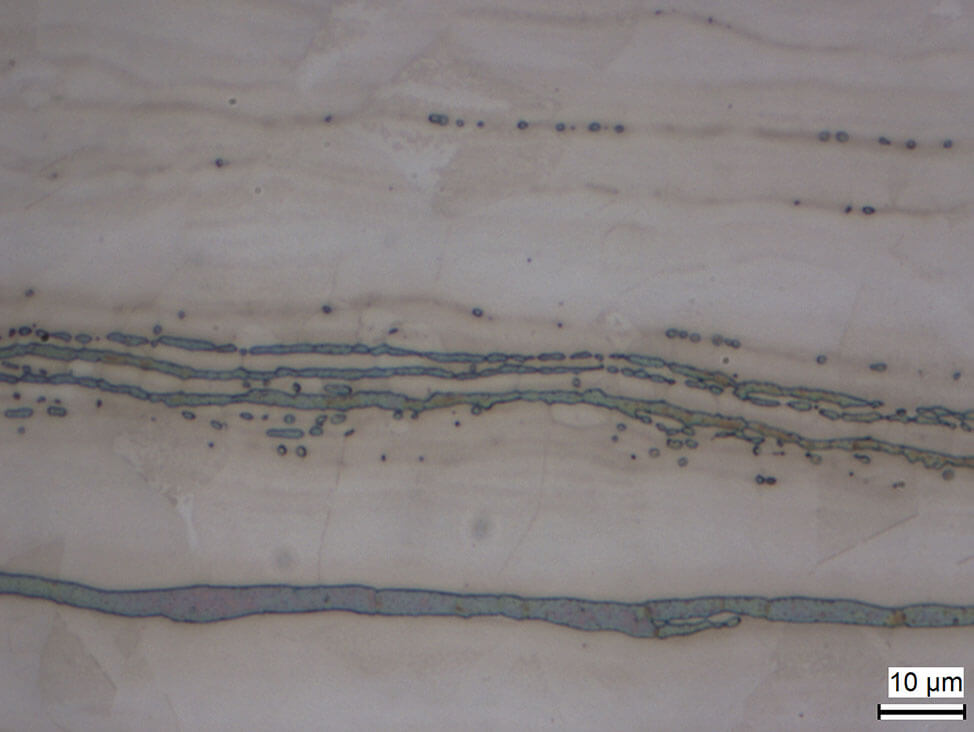 Fig. 10: Strings of deltaferrite in austenitic steel matrix, electrolytically etched with sodium hydroxide in water (20 %)
Austenitic-ferritic stainless steels
Fig. 10: Strings of deltaferrite in austenitic steel matrix, electrolytically etched with sodium hydroxide in water (20 %)
Austenitic-ferritic stainless steels (duplex) consist of ferrite and austenite. Electrolytic etching in a 20…40 % caustic soda solution reveals the structure, and the correct percentage of each phase can be estimated. These steels are ductile and are specifically used in the food, paper and petroleum industries.
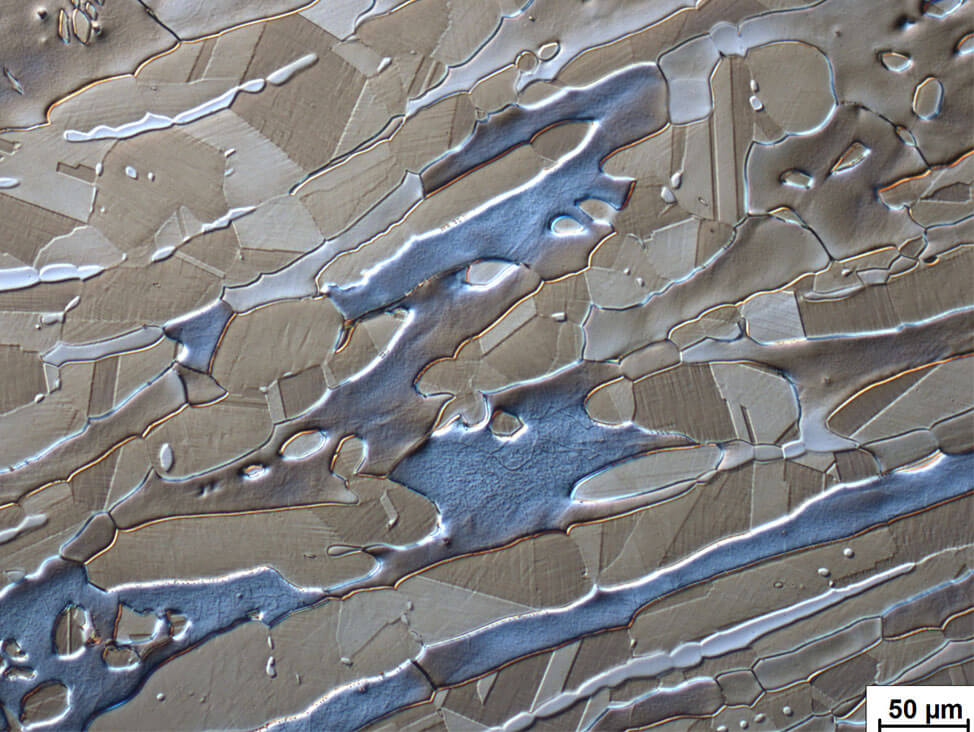 Fig. 11: Forged duplex steel showing blue ferrite, light to dark brown austenite. Double electrolytical etching; first 10 % oxalic acid in water and second etch 20 % sodium hydroxide in water; DIC
Fig. 11: Forged duplex steel showing blue ferrite, light to dark brown austenite. Double electrolytical etching; first 10 % oxalic acid in water and second etch 20 % sodium hydroxide in water; DIC